
|
so sleep alone tonight
My name is ღ||汇嘉||SOPHIAღ. Give me presents! On 22061996 :D [Cancer] [CHRISTIAN] PLMGSS 2.1 I love Aaron (: |
|
|
about this blog
This blog was opened by (SOPHIAARON) to accomodate (SOPHIA)'s Science journal.Do enjoy your stay here, and don't take what's not yours! tagboard
ShoutMix chat widget br> affiliates
DORCAS!!!(: MELISSA LISA LISHAN RACHEL RADIENT JANELLE JASLEY SEOWHWEE STELLA STACEY SARAH SHIYU JOYI Bue. Bue. Bue. archives
Videos. (:
-------------Physics------------Liquifaction. How to get out of a quicksand. Math Definitions : What Is Terminal Velocity. Newton's 3 laws of motion. Titration Technique using a burette How to use a pipette. Indroduction to Frictional force. Tacoma Bridge Collapsed! ---------------Chemistry---------- Forensic Video Periodic table song(: Crystal structures: graphite and diamond Chromotography- Separation of MIxtures Separation: Iron from Salt & Sand Mixture Steam Distillation Fractional Distillation Video Video Video Video Video Video Video Video Video Video Video Video Video credits
Design: doughnutcrazyIcon: morphine_kissed Do credit accordingly if you changed the icon. |
Biology 2- Raw food or cooked food?
So, does raw food or cooked food gets digested faster within us?Let' s do an in depth understanding of it first.   Firstly, the enzymes in the food gets destroyed by the heat. Which also means that the cooked food has much lesser enzymes as compared to raw food. Most raw food, like our bodies, is very perishable. When raw foods are exposed to temperatures above 118 degrees, they start to rapidly break down, just as our bodies would if we had a fever that high. One of the constituents of foods which can break down are enzymes. Enzymes help us digest our food. Enzymes are proteins though, and they have a very specific 3-dimensional structure in space. Once they are heated much above 118 degrees, this structure can change. Once enzymes are exposed to heat, they are no longer able to provide the function for which they were designed. Cooked foods contribute to chronic illness, because their enzyme content is damaged and thus requires us to make our own enzymes to process the food. The digestion of cooked food uses valuable metabolic enzymes in order to help digest your food. Digestion of cooked food demands much more energy than the digestion of raw food. In general, raw food is so much more easily digested that it passes through the digestive tract in 1/2 to 1/3 of the time it takes for cooked food. Eating enzyme-dead foods places a burden on your pancreas and other organs and overworks them, which eventually exhausts these organs. Many people gradually impair their pancreas and progressively lose the ability to digest their food after a lifetime of ingesting processed foods. Raw foods are rich in enzymes. Enzymes are needed for the digestive system to work. They are necessary to break down food particles so they can be utilized for energy. The human body makes approximately 22 different digestive enzymes which are capable of digesting carbohydrates, protein and fats. Raw vegetables and raw fruit are rich sources of enzymes.  So should we eat raw or cooked food??????? ---> Therefore, raw food is definitely much better than cooked food! Cooked food causes many types of chronic diseases while raw food doesn't! That definitely explains why animals don't get chronic diseases like cancer as often as we do. :P Biology 1 - Biuret's Test
Biuret TestFirstly, the purpose of this experiment is to test for the presence of protein the the food sample. We were give food sample X. (milk) These are the instructions for the experiment: 1) Add 5 to 10 drops of Biuret solution to the food sample. 2) Shake the sample well, after each drop. If the result is positive, which means that protein is present, the Biuret solution will turn from blue to violet colouration. So, what exactly is The Biuret Test? The biuret test is a chemical test used for detecting the presence of peptide bonds. In the presence of peptides, a copper(II) ion forms a violet-colored complex in an alkaline solution. Several variants on the test have been developed. The Biuret reaction can be used to assay the concentration of proteins because (for most proteins) peptide bonds occur with approximately the same frequency per gram of material. The intensity of the color, and hence the absorption at 540 nm, is directly proportional to the protein concentration, according to the Beer-Lambert law. In spite of its name, the reagent does not in fact contain biuret ((H2N-CO-)2NH). The test is so named because it also gives a positive reaction to the peptide bonds in the biuret molecule. Biuret reagentThe biuret reagent is made of potassium hydroxide (KOH) and hydrated copper (II) sulfate, together with potassium sodium tartrate. The reagent turns from blue to violet in the presence of proteins, blue to pink when combined with short-chain polypeptides. taken from: http://en.wikipedia.org/wiki/Biuret_test Physics 35
Atmospheric Pressure.  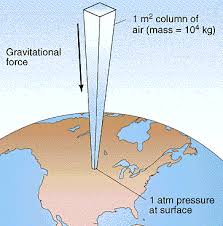 Atmospheric pressure is the force per unit area exerted against a surface by the weight of air above that surface in the Earth's atmosphere. In most circumstances atmospheric pressure is closely approximated by the hydrostatic pressure caused by the weight of air above the measurement point. Low pressure areas have less atmospheric mass above their location, whereas high pressure areas have more atmospheric mass above their location. Similarly, as elevation increases there is less overlying atmospheric mass, so that pressure decreases with increasing elevation. A column of air one square inch in cross-section, measured from sea level to the top of the atmosphere, would weigh approximately 14.7 lbf (65 N). Physics 34
Why does heat travel from Higher temperature to Lower temperature?Firstly, to understand this, we have to understand how heat travel. Heat can travel in 3 ways. First, Conduction, then Convection and Radiation. Both conduction and convection needs a medium while radiation, can travel through vacuum. What is conduction then? Conduction occurs when two object at different temperatures are in contact with each other. Heat flows from the warmer to the cooler object until they are both at the same temperature. Conduction is the movement of heat through a substance by the collision of molecules. At the place where the two object touch, the faster-moving molecules of the warmer object collide with the slower moving molecules of the cooler object. As they collide, the faster molecules give up some of their energy to the slower molecules. The slower molecules gain more thermal energy and collide with other molecules in the cooler object. This process continues until heat energy from the warmer object spreads throughout the cooler object. Some substances conduct heat more easily than others. Solids are better conductor than liquids and liquids are better conductor than gases. Metals are very good conductors of heat, while air is very poor conductor of heat. You experience heat transfer by conduction whenever you touch something that is hotter or colder than your skin e.g. when you wash your hands in warm or cold water. CONVECTION:
In liquids and gases, convection is usually the most efficient way to transfer heat. Convection occurs when warmer areas of a liquid or gas rise to cooler areas in the liquid or gas. As this happens, cooler liquid or gas takes the place of the warmer areas which have risen higher. This cycle results in a continous circulation pattern and heat is transfered to cooler areas. You see convection when you boil water in a pan. The bubbles of water that rise are the hotter parts of the water rising to the cooler area of water at the top of the pan. You have probably heard the expression "Hot air rises and cool air falls to take its place" - this is a description of convection in our atmosphere. Heat energy is transfered by the circulation of the air. RADIATION: Both conduction and convection require matter to transfer heat. Radiation is a method of heat transfer that does not rely upon any contact between the heat source and the heated object. For example, we feel heat from the sun even though we are not touching it. Heat can be transmitted though empty space by thermal radiation. Thermal radiation (often called infrared radiation) is a type electromagnetic radiation (or light). Radiation is a form of energy transport consisting of electromagnetic waves traveling at the speed of light. No mass is exchanged and no medium is required. Objects emit radiation when high energy electrons in a higher atomic level fall down to lower energy levels. The energy lost is emitted as light or electromagnetic radiation. Energy that is absorbed by an atom causes its electrons to "jump" up to higher energy levels. All objects absorb and emit radiation. ( Here is a java applet showing how an atom absorbs and emits radiation) When the absorption of energy balances the emission of energy, the temperature of an object stays constant. If the absorption of energy is greater than the emission of energy, the temperature of an object rises. If the absorption of energy is less than the emission of energy, the temperature of an object falls. So, the reason why heat travels from a region of higher temperature to a region of lower temperature is because there's a difference between two temperature. This falls into the category of thermal physics. Scientists make use of this law to construct the way hospitals are now. Do you notice that the corridors of the hospitals are always cooler than the wards? It makes use of the law of convection current. When the ward door is open, the cold air rushes in, hence pushing the virus and bacteria that was in the room into the room once again. This prevents others from contracting the virus. Sources: http://coolcosmos.ipac.caltech.edu/cosmic_classroom/light_lessons/thermal/transfer.html |